Currently, metabolic disorders have become a major global public health challenge, posing a serious threat to socioeconomic development. In China, the number of adults with diabetes exceeds 141 million, the highest in the world; meanwhile, the prevalence of metabolic-associated fatty liver disease (MAFLD) among adults has reached an alarming 44.3%, affecting nearly 460 million individuals. The progression of diabetesfatty liver disease can lead to cardiovascularcerebrovascular complications, with vascular calcification being a major cause of disabilitymortality. However, the underlying mechanisms of vascular calcification remain poorly understood,effective clinical strategies for preventiontreatment are still lacking.
On December 3, two original research papers on the pathogenesis of vascular calcification from Professor Liu Jianghua’s team at our hospital were simultaneously acceptedpublished by Advanced Science (Impact Factor: 14.3), a top-tier journal in the Chinese Academy of Sciences (CAS) Quartile 1. The first paper, titled “Bacteroides fragilis exacerbates T2D vascular calcification by secreting extracellular vesicles to induce M2 macrophages”, presents the findings by Dr. Jinsong Caocolleagues. The team demonstrated that the gut bacterium Bacteroides fragilis (BF) promotes the expression of Trib1 via the EV–STING–Mef2d signaling axis, inducing macrophages polarization towards the M2 phenotypeupregulating Serpine1 expression, thereby aggravating vascular calcification in type 2 diabetes (T2D). This study is the first to demonstrate that both BFperipheral M2-type monocytes/macrophages are significantly elevated in T2D patients with vascular calcification. Moreover, both BFits extracellular vesicles (EVs) were shown to enhance vascular calcification, whereas depletion of macrophages markedly attenuated BF-derived EV-induced vascular calcification.
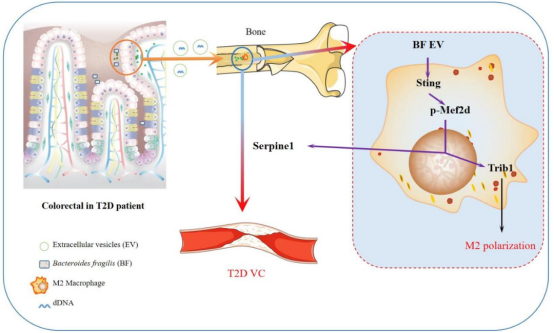
Figure 1. Bacteroides fragilis aggravates T2D-associated vascular calcification via the EV–STING–Mef2d pathway
The second paper, titled “Hepatic Steatosis Aggravates Vascular Calcification via Extracellular Vesicle-mediated Osteochondrogenic Switch of Vascular Smooth Muscle Cells”, presents the findings of Dr. Zhaolin Zengcolleagues. For the first time, the study reveals that in patients with metabolic-associated fatty liver disease (MAFLD), hepatocyte-derived EVs can transfer the functional protein LGALS3BP to promote osteogenic differentiation of vascular smooth muscle cells (VSMCs)foam cell formation, while inducing macrophage polarization toward the pro-inflammatory M1 phenotype. These processes collectively exacerbate vascular calcificationatherosclerosis. Furthermore, the study demonstrates that disrupting the EV-mediated crosstalk between steatotic hepatocytesvascular cells can attenuate the development of vascular calcification, offering new perspectivesbreakthroughs for its preventiontreatment.
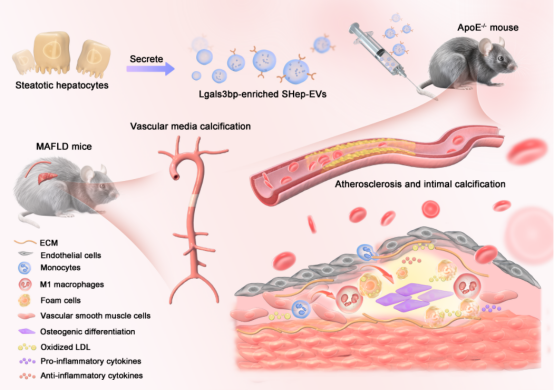
Figure 2. Hepatocyte-derived EVs promote vascular calcification via transfer of the functional protein LGALS3BP
Professor Jianghua Liu’s team has long been dedicated to investigating the pathogenesistherapeutic strategies for vascular calcification. They were among the first to establish a prospective research cohort for T2D patients with vascular calcification. Over the past decade, the team’s research has been published in internationally renowned journals such as Advanced Science, Brain, Behavior,Immunity,Metabolism. The group has led six projects funded by the National Natural Science Foundation of China, two projects funded by the Natural Science Foundation of Hunan Province,one major sciencetechnology project in Hunan Province. They have authored two academic monographs, contributed to a national guideline on the preventiontreatment of vascular calcification, won the Second Prize of the Hunan Provincial ScienceTechnology Progress Award,held one national invention patent.