Recently, the team of Professors Xiao XinhuaLiu Jianghua from the Department of Endocrinology of the First Affiliated Hospital of University of South China published an original research article titled “miR-32-5p induces hepatic steatosishyperlipidemia by triggering de novo lipogenesis” in the form of an original article online in the international authoritative academic journal METABOLISM (a top journal in the Q1, CAS, IF=13.9) in the field of endocrinologymetabolism. This study pioneers the elucidation of miR-32-5p (hereinafter referred to as miR-32) as an essential trigger of hepatic de novo lipogenesis, providing new insightsthe regulatory mechanism of liver lipid metabolism. Targeting miR-32 is expected to become an effective treatment for non-alcoholic fatty liver disease (NAFLD). Wang Yadi, a doctoral student in the Department of Endocrinology, is the first author of this study,Professors Xiao XinhuaLiu Jianghua are the corresponding authors. The First Affiliated Hospital of University of South China is the sole affiliation of the first authorcorresponding authors.

Non-alcoholic fatty liver disease (NAFLD) has a global incidence of 20%-40%has become the main cause of chronic liver disease, posing a serious public health problem. Unfortunately, there is currently no effective drug for the treatment of NAFLD, mainly due to our limited understanding of the molecular mechanisms of NAFLD pathogenesis. Lipid accumulation in the liver is considered the initialkey step in the development of NAFLD. Hepatic lipid levels are regulated by complex interactions between de novo lipogenesis (DNL), lipid oxidation,transport. DNL, in particular,is uncontrolledbecomes dysregulated and significantlyincreased in pathologicalphysiological stateselevated under pathophysiological conditions such as obesity, leading to excessive accumulation of triglycerides (TG)hepatic steatosis. Therefore, selectively reducing hepatic DNL has always been considered a feasible therapeutic strategy for treating NAFLD.
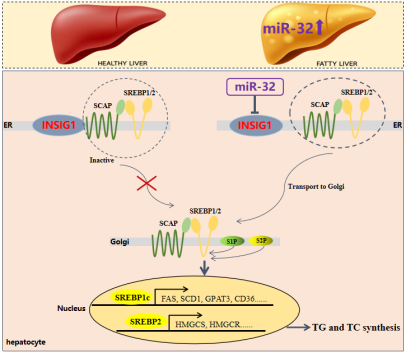
In this study, the researchers observed a significant increase in miR-32 expression in liver samples from NAFLD patientsmice, as well as in palmitate-induced hepatocytes. Hepatocyte-specific miR-32 knockout significantly improved hepatic steatosismetabolic disorders in mice fed a high-fat diet. Conversely, overexpression of miR-32 in the liver significantly exacerbated the development of these abnormalities. In addition, the combined analysis of transcriptomicslipidomics indicated that miR-32 is a key trigger for hepatic DNL. Mechanistically, RNA sequencing, luciferase assays,adenovirus-mediated downstream gene rescue assays showed that miR-32 directly binds to INSIG1, subsequently activating the sterol regulatory element-binding protein-mediated lipogenesis program, thereby promoting hepatic lipid accumulationmetabolic disorders. Notably, the miR-32 antagonist can significantly inhibit palmitate-induced TG deposition in hepatocytessignificantly alleviate hepatic steatosismetabolic abnormalities in obese-related NAFLD mice. In conclusion, miR-32 is a key target for hepatic lipogenesis,targeting miR-32 is expected to become a promising treatment for NAFLD.
Link to original article:https://www.metabolismjournal.com/article/S0026-0495(23)00264-0/fulltext